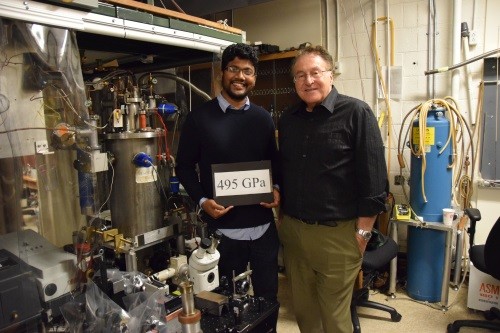
Pressure experts: Isaac Silvera (right) together with Ranga Dias.
By Michael Banks
A team led by Isaac Silvera at Harvard University hit the headlines earlier this year when it claimed to have been the first to create metallic hydrogen. Silvera, who will be giving an invited talk about metallic hydrogen at the Chinese Physical Society meeting at Sichuan University (7–9 September), outlines the challenges in working with the material and what future applications it may have. The event is co-sponsored by the Institute of Physics, which publishes Physics World.
What is metallic hydrogen?
Metallic hydrogen is a metal that is made entirely of hydrogen atoms. On Earth, hydrogen exists naturally as a gas. When cooled to 20.3 K it liquefies and at 14 K becomes a molecular solid. In 1935 Eugene Wigner and Hillard Huntington at Princeton University theorized that a molecular hydrogen lattice will dissociate to an atomic hydrogen lattice at pressures of 25 GPa, allowing electrons to flow freely through it, therefore creating metallic hydrogen. Modern calculations, however, predict that you need pressures of around 400–500 GPa to create it.
Physicists have spent decades trying to make it, so what allowed it to be done now?
At low temperatures, enormous pressures – until recently, around 350 GPa – have been achieved in diamond anvil cells (DACs). In DACs, the hydrogen is in a hole or cavity in a metallic gasket that is pressed between two diamond anvils. We developed techniques to achieve even higher pressures – touching 500 GPa. It was not a new method, but recognizing that certain existing procedures resulted in the diamonds failing and therefore limiting the pressure. To reach higher pressures required, for example, preventing the diffusion of hydrogen into the diamonds, which makes them brittle, as well as assuring the perfect alignment of the DACs during pressurization.
What are the challenges when working with metallic hydrogen?
The main challenge is creating the enormous pressures that are required. In DACs, the highest pressures are achieved with a small culet. The culet is the flat-polished part on the tip of a brilliant-cut diamond. At the highest pressures, we use culet flats around 20–30 µm in diameter with a hydrogen sample size of about 10 µm in diameter and 1 µm thick. While the sample is in a cryostat, this requires careful study using microscopes that creates certain optical challenges. Furthermore, the hydrogen sample is between two diamond anvils, and to study the sample, radiation must pass through those diamonds. The diamonds have a region in the visible and infrared where they are transparent, but block out light in the ultra violet, which limits studying them in this region of the spectrum.
Why is there still some scepticism that metallic hydrogen has been created in the lab?
Several groups around the world are working on this challenge. Yet they are continuing to use the same old techniques and achieving the same pressures. Thus, criticisms have been mainly aimed at our methods to determine the pressure. Conventional techniques to determine the pressure use Raman scattering in the highly stressed region of the diamond anvil. But it is known that illuminating stressed diamonds with lasers can lead to them failing. We avoided using this method until we achieved and determined that the hydrogen was metallic by measuring its reflectance. We then used a low-laser power of around 20 mW to measure the pressure with Raman scattering and succeeded.
How long can you isolate a sample for?
Interestingly, we maintained the metallic hydrogen in our cryostat for about three months before again attempting to measure pressure by illuminating it with a laser. We used 1/20th of the laser intensity – 0.5 mW – and the diamonds immediately failed. We believe that the highly stressed diamonds relax by creating defects in their lattice and these defects interact with the light.
What possible uses could metallic hydrogen have?
Metallic hydrogen may be a room-temperature superconductor. It may also be metastable – that is, it may remain metallic hydrogen even when the pressure is lifted. If so, and if it is a room-temperature superconductor, it would be revolutionary. Theoretically, it is also the most powerful rocket propellant known to man, and if metastable and could be produced in large quantities, it would transform rocketry.
What are you currently working on?
We are repeating our first experiment to make metallic hydrogen and measure its reflectance. We then plan to measure its electrical conductivity to determine if it is a room-temperature superconductor. We will also aim to test if it is metastable as well as study isotope effects, such as the metallization of deuterium.
• A feature about the quest for metallic hydrogen will appear in the October 2017 issue of Physics World. The APS–CPS–IOP Joint International Session on Materials and Physics Under Extreme Conditions takes place on 9 September at the CPS Fall Meeting.
Guidelines
Show/hide formatting guidelines
this text was deletedwhere people live in harmony with nature and animals</q>
Some text